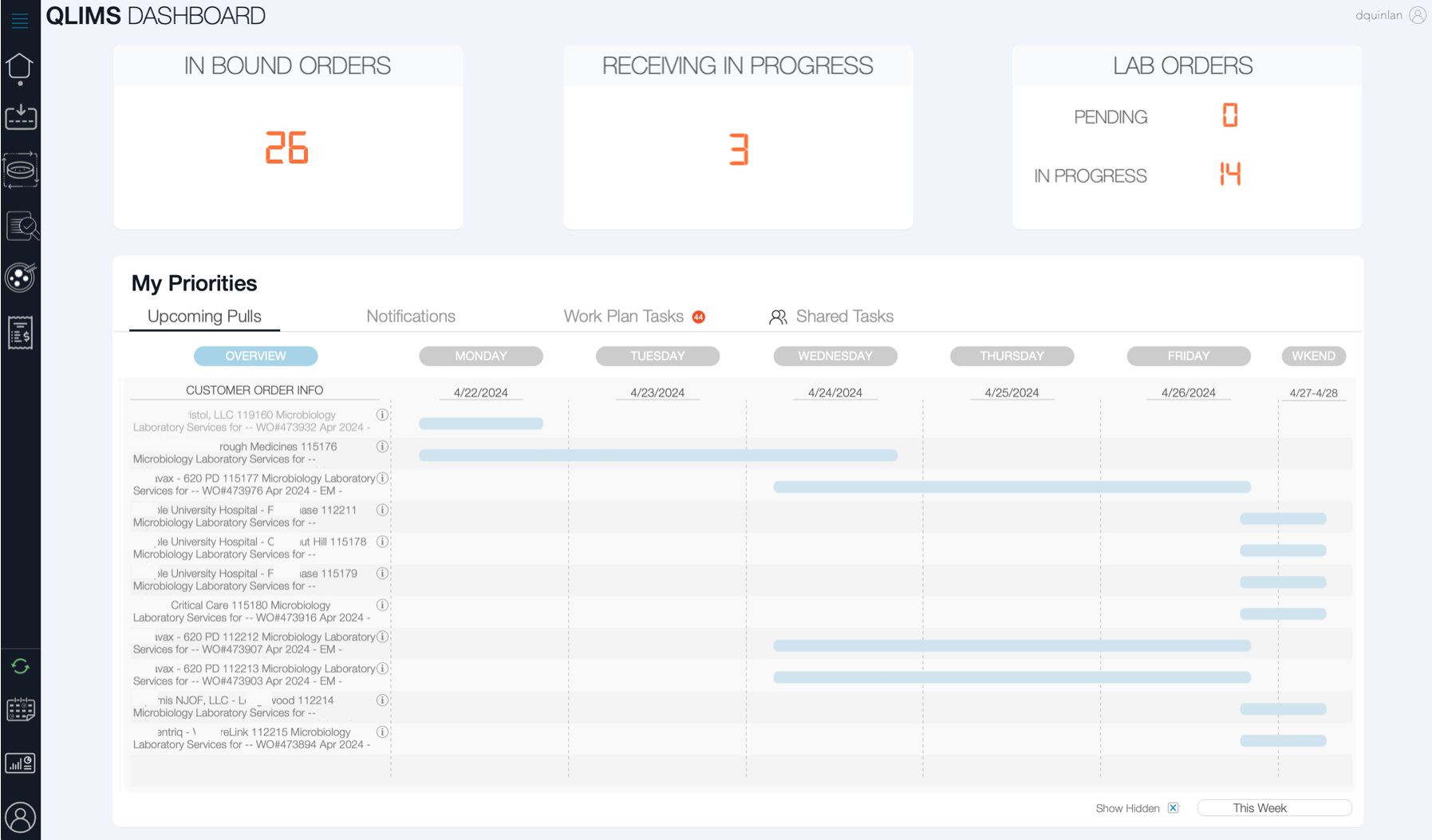
Our laboratory is ISO/IEC 17025:2017 accredited, signifying our commitment to maintaining the highest standards of quality and accuracy. We utilize state-of-the-art technology such as the MALDI Biotyper® sirius GP and have advanced systems like our QLIMS software for efficient sample management. Our facilities are designed to guarantee uninterrupted service with backup power systems and HEPA-filtered air supply.
Quantus distinguishes itself by offering scalable solutions that grow with your needs, rapid results reporting, and a comprehensive organism library for extensive coverage. Our One Stop Shop model allows for streamlined third-party services, enhancing operational efficiency by consolidating services and minimizing the need for multiple vendors.
Absolutely. Our facilities include large-scale incubation capacities with emergency power backup, ensuring reliable and continuous service for projects of any size. We are equipped to handle high-throughput demands, offering tailored services that can adapt to the most challenging and extensive microbiology testing requirements.
We provide microbiology testing services compliant with GMP, USP, and FDA standards. Our tests include, but are not limited to, microbial environmental monitoring, organism identification, media fill testing for aseptic processes, and water quality testing. Each service is designed to meet rigorous validation and compliance needs, ensuring that your products and processes adhere to all necessary regulatory guidelines.
 Social icons
Social icons